Lithium-Ion Battery vs Lead Acid Battery: A Comprehensive Comparison
1. Introduction
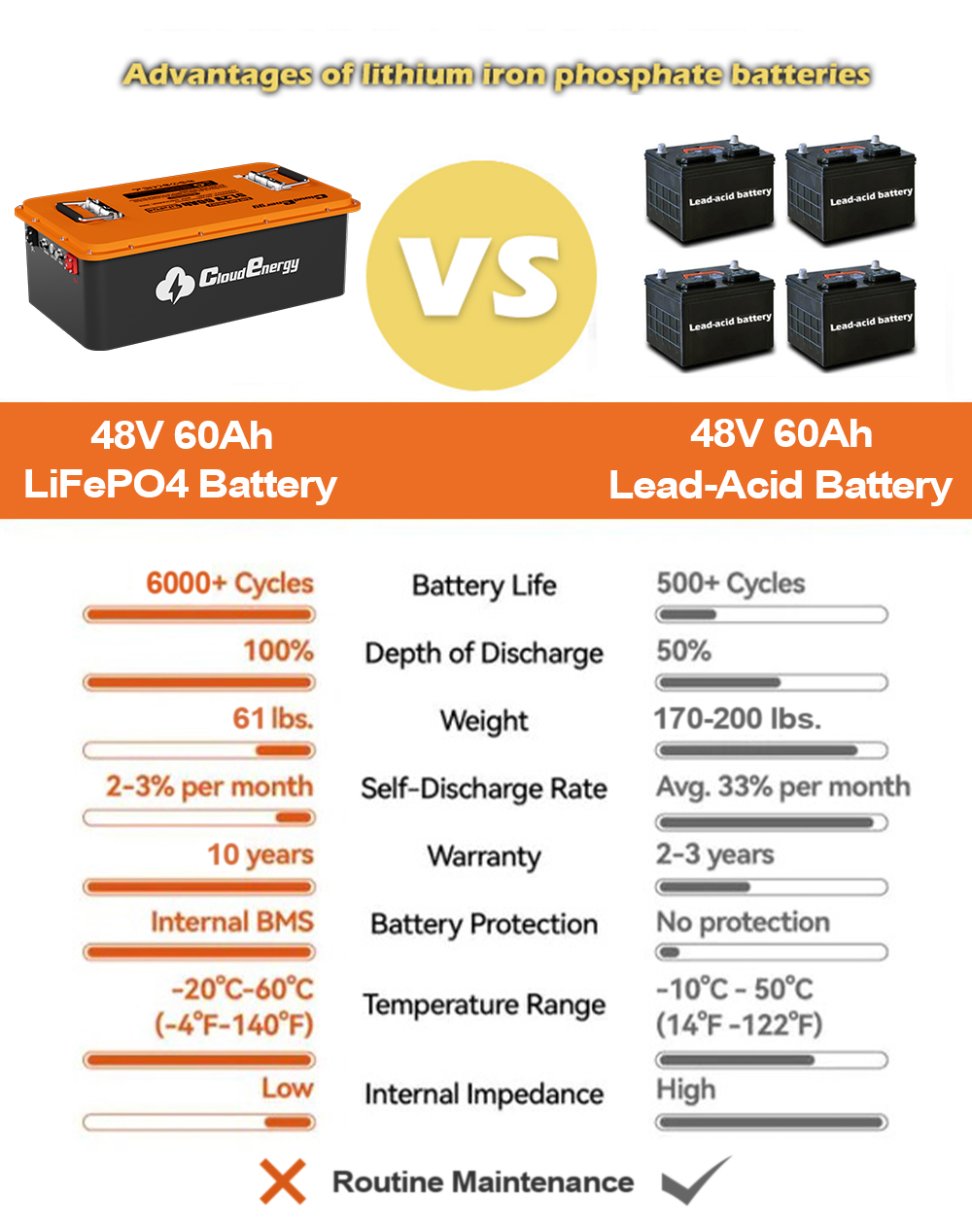
1.1 Overview of Battery Technologies
In the realm of energy storage, batteries play a pivotal role in powering a myriad of devices, from consumer electronics to electric vehicles and renewable energy systems. Among the various battery technologies available, lithium-ion and lead-acid batteries are two of the most widely used. Each technology has its unique characteristics, advantages, and disadvantages, making the choice between them critical for specific applications.
1.2 Importance of Battery Selection
Selecting the appropriate battery technology is essential for optimizing performance, cost-effectiveness, and longevity. As industries increasingly shift towards sustainable energy solutions, understanding the differences between lithium-ion and lead-acid batteries becomes paramount. This article delves into the composition, advantages, disadvantages, and applications of both battery types, providing a comprehensive comparison to aid in informed decision-making.
2. Lithium-Ion Batteries
2.1 Composition and Chemistry
Lithium-ion batteries are composed of lithium compounds, typically lithium cobalt oxide or lithium iron phosphate, serving as the cathode, while graphite is used for the anode. The electrolyte consists of a lithium salt dissolved in an organic solvent, facilitating the movement of lithium ions between the electrodes during charge and discharge cycles. This electrochemical process allows lithium-ion batteries to store and release energy efficiently.
2.2 Advantages
2.2.1 Energy Density
One of the most significant advantages of lithium-ion batteries is their high energy density. They can store more energy in a smaller and lighter package compared to lead-acid batteries. This characteristic makes them ideal for applications where space and weight are critical, such as in electric vehicles and portable electronics.
2.2.2 Cycle Life
Lithium-ion batteries boast a longer cycle life, typically ranging from 2,000 to 5,000 charge-discharge cycles, depending on usage and conditions. This longevity translates to fewer replacements over time, making them a more sustainable choice in the long run.
2.2.3 Weight and Size
The lightweight and compact design of lithium-ion batteries further enhances their appeal. Their reduced weight is particularly advantageous in applications like electric vehicles, where every kilogram counts towards efficiency and performance.
2.3 Disadvantages
2.3.1 Cost
Despite their advantages, lithium-ion batteries come with a higher upfront cost compared to lead-acid batteries. The manufacturing process and materials used contribute to this expense, making them less accessible for budget-conscious applications.
2.3.2 Safety Concerns
Lithium-ion batteries can pose safety risks, including thermal runaway, which can lead to fires or explosions if not managed properly. This necessitates the incorporation of sophisticated battery management systems to monitor and control charging and discharging processes.
3. Lead Acid Batteries
3.1 Composition and Chemistry
Lead-acid batteries consist of lead dioxide (PbO2) and sponge lead (Pb) plates submerged in a sulfuric acid electrolyte. The electrochemical reactions between these materials generate electrical energy. This technology has been in use for over a century, making it one of the most established battery technologies available.
3.2 Advantages
3.2.1 Cost-Effectiveness
Lead-acid batteries are generally more affordable than lithium-ion batteries, making them a popular choice for applications where cost is a primary concern. Their lower initial investment can be appealing for industries with tight budgets.
3.2.2 Reliability
Known for their robustness, lead-acid batteries can withstand harsh environmental conditions and deep discharges. Their reliability has made them a staple in various applications, including automotive and backup power systems.
3.2.3 Established Technology
The long history of lead-acid batteries means that they are widely understood and supported by a vast infrastructure. This established technology benefits from a well-developed recycling process, contributing to their sustainability.
3.3 Disadvantages
3.3.1 Weight
Lead-acid batteries are significantly heavier than their lithium-ion counterparts, which can be a disadvantage in applications where weight is a critical factor. Their bulkiness can also limit their use in portable devices.
3.3.2 Shorter Cycle Life
The cycle life of lead-acid batteries is considerably shorter, typically ranging from 300 to 1,500 cycles. This limitation necessitates more frequent replacements, which can increase long-term costs despite their lower initial price.
4. Comparison of Performance
4.1 Energy Efficiency
Lithium-ion batteries exhibit higher energy efficiency, with efficiencies around 95%, compared to lead-acid batteries, which typically range from 80% to 85%. This efficiency translates to faster charging times and more effective energy utilization.
4.2 Lifespan and Maintenance
Lithium-ion batteries require minimal maintenance and have a longer lifespan, while lead-acid batteries necessitate regular maintenance, including electrolyte level checks and equalization charging. The longer lifespan of lithium-ion batteries can offset their higher initial costs over time.
4.3 Environmental Impact
Both battery types have environmental considerations. Lead-acid batteries are highly recyclable, but improper disposal can lead to environmental hazards due to lead and sulfuric acid. Lithium-ion batteries, while less toxic, require careful recycling processes to recover valuable materials and prevent environmental harm.
5. Applications
5.1 Use Cases for Lithium-Ion Batteries
Lithium-ion batteries are extensively used in applications requiring high energy density and lightweight solutions, including:
-
Electric vehicles (EVs) and hybrid electric vehicles (HEVs)
-
Consumer electronics (smartphones, laptops, tablets)
-
Renewable energy storage systems (solar and wind)
-
Aerospace applications (satellites and drones)
5.2 Use Cases for Lead Acid Batteries
Lead-acid batteries are commonly found in applications where cost-effectiveness and reliability are paramount, such as:
-
Automotive starting, lighting, and ignition (SLI) systems
-
Uninterruptible power supply (UPS) systems
-
Backup power for telecommunications
-
Forklifts and material handling equipment
6. Conclusion
6.1 Summary of Key Points
In summary, both lithium-ion and lead-acid batteries have distinct advantages and disadvantages that make them suitable for different applications. Lithium-ion batteries excel in energy density, cycle life, and weight, making them ideal for modern technology and electric vehicles. Conversely, lead-acid batteries offer cost-effectiveness, reliability, and established technology, making them a viable choice for traditional applications.
6.2 Future Trends in Battery Technology
As technology advances, the battery landscape is evolving. Innovations in lithium-ion technology, such as solid-state batteries, promise to enhance safety and performance further. Meanwhile, ongoing research into lead-acid battery improvements aims to extend their lifespan and efficiency. Understanding these trends will be crucial for stakeholders in selecting the most appropriate battery technology for their needs.
In conclusion, the choice between lithium-ion and lead-acid batteries ultimately depends on specific application requirements, budget constraints, and performance expectations. By carefully considering these factors, users can make informed decisions that align with their energy storage needs.
Leave a Reply
- Golf Cart Lithium Battery Replacement for 2013 Club Car Precedent
- Upgrade Your Club Car DS with CloudEnergy Lithium Batteries
- Understanding LiFePO4 Charge Curves: A Comprehensive Guide
- Upgrading Your Golf Cart to Lithium Batteries: A Comprehensive Guide
- What Are the Best Battery Types for Off-Grid Living?